Kind of bond in chemistry crossword, a topic that has intrigued and captivated the minds of scientists and students alike, unveils the intricate tapestry of chemical interactions that govern the formation and behavior of matter. Delving into this fascinating realm, we embark on a journey to unravel the diverse types of chemical bonds, their strengths, weaknesses, and the molecules they hold together.
From the fundamental covalent bonds that unite atoms to form molecules, to the electrostatic attraction of ionic bonds and the cooperative nature of metallic bonds, we explore the spectrum of chemical bonding. Hydrogen bonds, with their unique ability to influence molecular structure and properties, and van der Waals forces, the subtle interactions that contribute to the behavior of gases and liquids, complete our investigation into the captivating world of chemical bonding.
Chemical Bonds in Chemistry
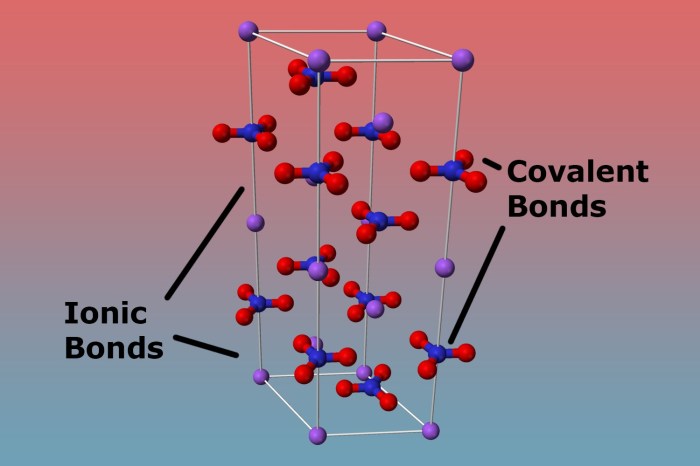
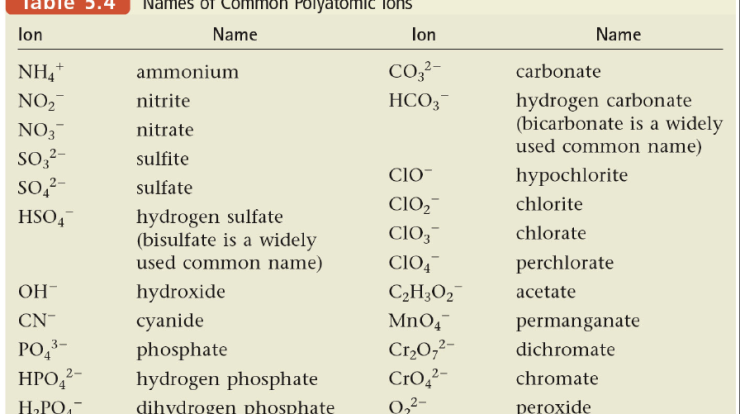
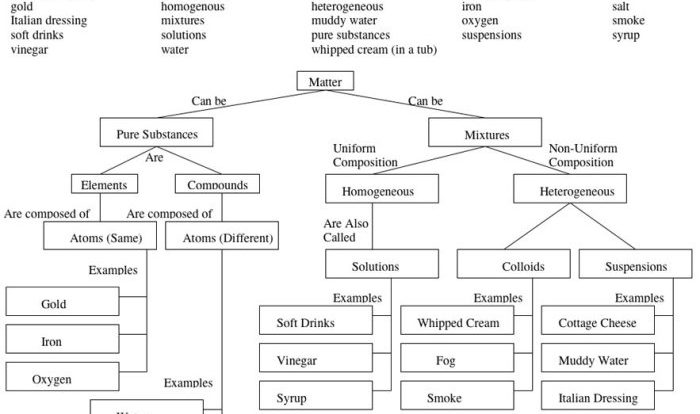
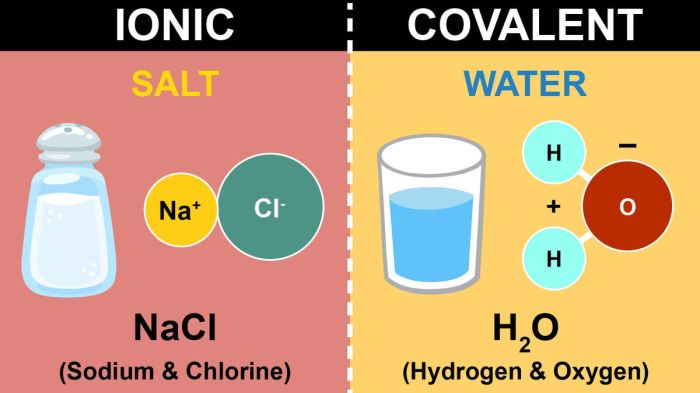
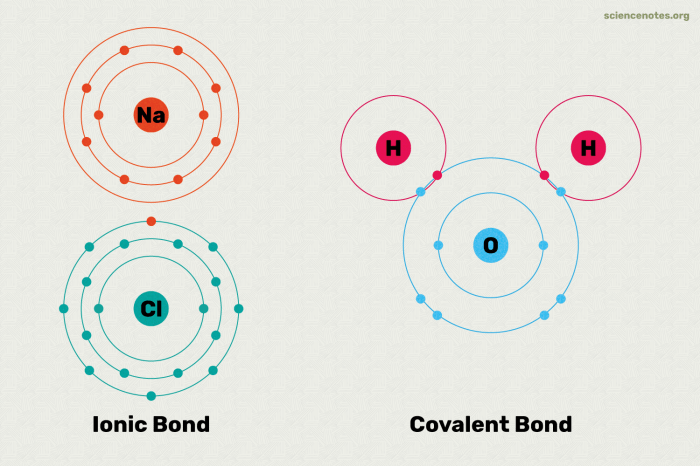
Chemical bonds are the forces that hold atoms together to form molecules and compounds. There are three main types of chemical bonds: covalent bonds, ionic bonds, and metallic bonds.Covalent bonds are formed when two atoms share one or more pairs of electrons.
The strength of a covalent bond depends on the number of shared electrons. The more shared electrons, the stronger the bond. Covalent bonds are typically found in molecules formed between nonmetals.Ionic bonds are formed when one atom transfers one or more electrons to another atom.
The atom that loses electrons becomes a positively charged ion, and the atom that gains electrons becomes a negatively charged ion. The oppositely charged ions are attracted to each other by electrostatic forces. Ionic bonds are typically found in compounds formed between metals and nonmetals.Metallic
bonds are formed when the atoms in a metal share their valence electrons in a sea of electrons. The valence electrons are not attached to any particular atom, but they are free to move throughout the metal. Metallic bonds are typically found in metals.The
strength of a chemical bond depends on the type of bond, the number of atoms involved in the bond, and the electronegativity of the atoms involved. Electronegativity is a measure of an atom’s ability to attract electrons. The more electronegative an atom, the more strongly it will attract electrons.Chemical
bonds are essential for the formation of molecules and compounds. They determine the properties of molecules and compounds, such as their shape, size, and reactivity.
Covalent Bonds
A covalent bond is a chemical bond that involves the sharing of electron pairs between atoms. These electron pairs are known as bonding pairs, and the stable balance of attractive and repulsive forces between atoms, when they share electrons, is known as covalent bonding.
Formation of Covalent Bonds
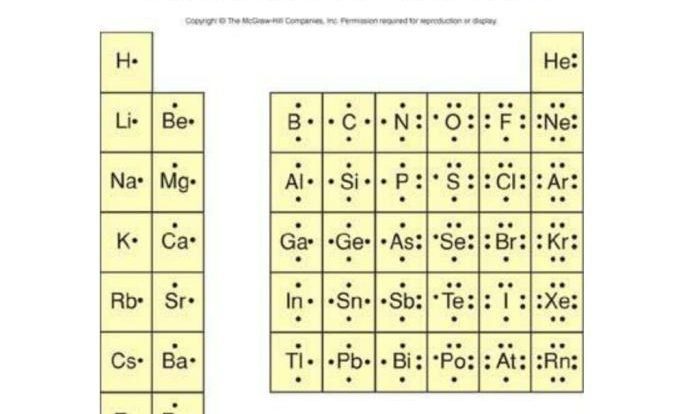
Covalent bonds are formed when atoms share one or more pairs of electrons. The shared electrons are attracted to the nuclei of both atoms, creating a force that holds the atoms together. The number of covalent bonds that an atom can form depends on the number of valence electrons it has.
Valence electrons are the electrons in the outermost shell of an atom, and they are the electrons that participate in chemical bonding.
Examples of Covalent Bonds
Covalent bonds are found in a wide variety of molecules, including:
- Water (H 2O)
- Methane (CH 4)
- Ammonia (NH 3)
- Carbon dioxide (CO 2)
- Glucose (C 6H 12O 6)
Covalent bonds are the strongest type of chemical bond, and they are responsible for holding atoms together in molecules.
Ionic Bonds
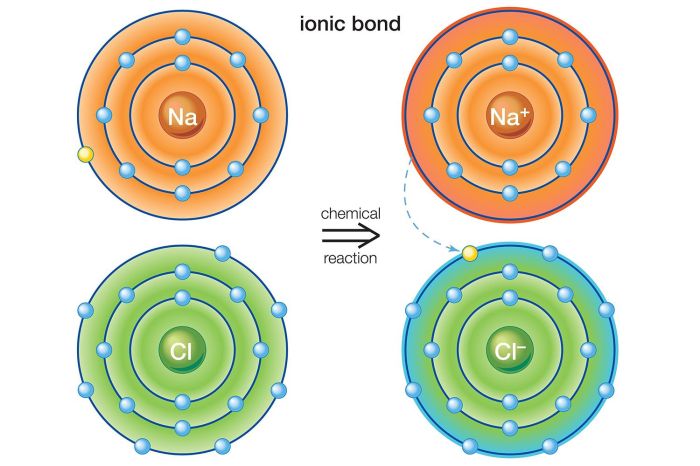
Ionic bonds are a type of chemical bond formed between two oppositely charged ions. These bonds are formed when one atom transfers one or more electrons to another atom, resulting in the formation of positive and negative ions.
Ionic bonds are typically formed between a metal and a non-metal. The metal atom loses one or more electrons, forming a positively charged ion (cation), while the non-metal atom gains the electrons, forming a negatively charged ion (anion). The oppositely charged ions are then attracted to each other by electrostatic forces, forming an ionic bond.
Examples of Ionic Compounds
- Sodium chloride (NaCl): Sodium (Na) loses one electron to chlorine (Cl), forming Na+ and Cl- ions, which are held together by an ionic bond.
- Potassium iodide (KI): Potassium (K) loses one electron to iodine (I), forming K+ and I- ions, which are held together by an ionic bond.
- Calcium oxide (CaO): Calcium (Ca) loses two electrons to oxygen (O), forming Ca2+ and O2- ions, which are held together by an ionic bond.
Hydrogen Bonds

A hydrogen bond is an attractive interaction between a hydrogen atom from one molecule and an electronegative atom, such as oxygen, nitrogen, or fluorine, from another molecule.
Hydrogen bonds are formed when a hydrogen atom is covalently bonded to a highly electronegative atom, such as oxygen, nitrogen, or fluorine. This creates a partial positive charge on the hydrogen atom and a partial negative charge on the electronegative atom.
The partial positive charge on the hydrogen atom can then interact with the partial negative charge on the electronegative atom of another molecule, forming a hydrogen bond.
Examples of Molecules Held Together by Hydrogen Bonds
- Water (H2O)
- Ammonia (NH3)
- Methanol (CH3OH)
- Ethanol (C2H5OH)
- DNA
- Proteins
Metallic Bonds
Metallic bonds are the attractive forces that hold metal atoms together in a metallic structure. These bonds arise from the electrostatic attraction between positively charged metal ions and the surrounding sea of mobile electrons.
Formation of Metallic Bonds
When a metal atom loses one or more electrons, it becomes a positively charged metal ion. These metal ions are then surrounded by a cloud of delocalized electrons that are free to move throughout the metal. The attraction between the positively charged metal ions and the negatively charged electron cloud holds the metal atoms together in a metallic bond.
Examples of Metals Held by Metallic Bonds
Metals that are held together by metallic bonds include:
- Sodium (Na)
- Potassium (K)
- Calcium (Ca)
- Magnesium (Mg)
- Aluminum (Al)
- Iron (Fe)
- Copper (Cu)
- Silver (Ag)
- Gold (Au)
van der Waals Forces
van der Waals forces are weak intermolecular forces that act between molecules. They are caused by the temporary fluctuations in the electron distribution of molecules, which create instantaneous dipoles. These dipoles can then interact with each other, creating a weak attractive force.van
der Waals forces are typically much weaker than covalent bonds, ionic bonds, or hydrogen bonds. However, they can be significant in determining the properties of molecules, such as their melting point and boiling point.
Types of van der Waals Forces
There are three main types of van der Waals forces:
- London dispersion forcesare caused by the temporary fluctuations in the electron distribution of molecules. These forces are always present, even between nonpolar molecules.
- Dipole-dipole forcesare caused by the interaction between permanent dipoles in molecules. These forces are stronger than London dispersion forces, but they are only present between polar molecules.
- Hydrogen bondingis a special type of dipole-dipole force that occurs between molecules that contain hydrogen atoms bonded to highly electronegative atoms, such as oxygen or nitrogen. Hydrogen bonding is stronger than both London dispersion forces and dipole-dipole forces.
Examples of Molecules Held Together by van der Waals Forces, Kind of bond in chemistry crossword
van der Waals forces are responsible for the cohesion of many substances, including:
- Noble gases
- Nonpolar molecules, such as methane and ethane
- Polar molecules, such as water and ammonia
Detailed FAQs: Kind Of Bond In Chemistry Crossword
What is the strongest type of chemical bond?
Covalent bonds are generally the strongest type of chemical bond.
What type of bond is formed between a metal and a non-metal?
Ionic bonds are formed between a metal and a non-metal.
What is the difference between a covalent bond and an ionic bond?
Covalent bonds involve the sharing of electrons between atoms, while ionic bonds involve the transfer of electrons from one atom to another.