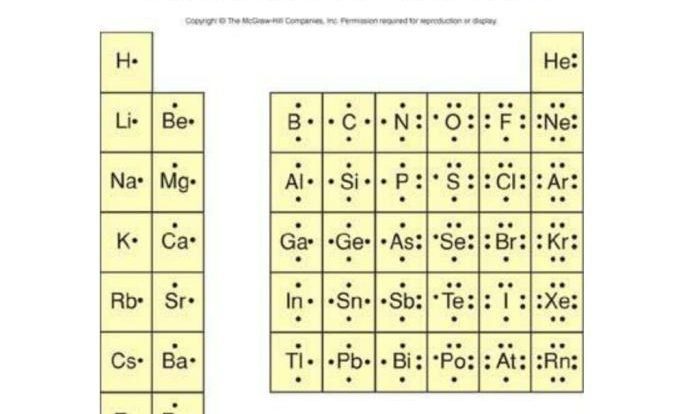
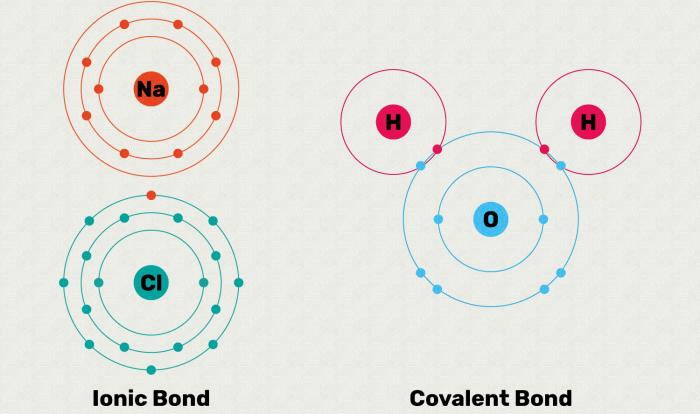
The classification of matter POGIL answer key PDF provides a comprehensive resource for students studying the fundamental concepts of matter. This guide offers a clear and concise explanation of the three states of matter, mixtures, solutions, physical and chemical changes, chemical reactions, and the periodic table.
With its in-depth explanations and illustrative examples, this answer key empowers students to grasp the complexities of matter classification, enabling them to excel in their academic pursuits.
Classification of Matter: Key Concepts
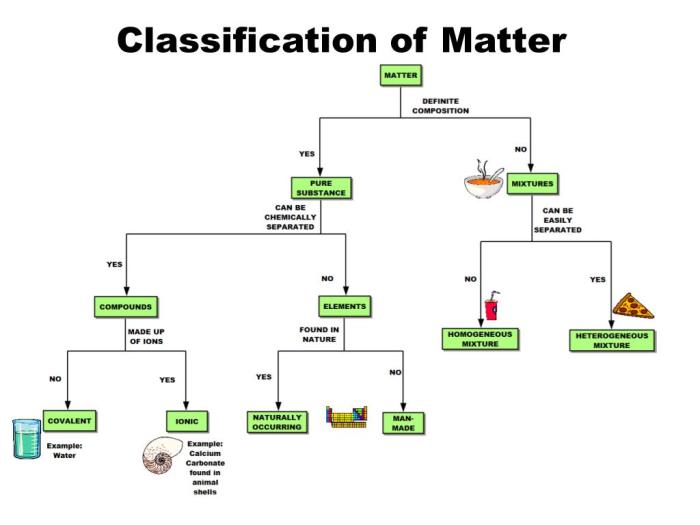
Matter can exist in three states: solid, liquid, and gas. Each state has distinct properties. Solids have a definite shape and volume, liquids have a definite volume but no definite shape, and gases have no definite shape or volume.Mixtures are combinations of two or more substances that are not chemically bonded.
Solutions are mixtures in which the components are evenly distributed. Homogeneous mixtures have the same composition throughout, while heterogeneous mixtures have different compositions in different parts.
Classification of Matter: Physical and Chemical Changes

Physical changes involve changes in the physical properties of a substance, such as its shape, size, or state. Chemical changes involve changes in the chemical composition of a substance.Examples of physical changes include melting, freezing, boiling, and sublimation. Examples of chemical changes include burning, rusting, and digestion.Physical
changes are usually reversible, while chemical changes are usually irreversible.
Classification of Matter: Chemical Reactions
A chemical reaction is a process in which one or more substances are transformed into one or more different substances.The law of conservation of mass states that the total mass of the reactants in a chemical reaction is equal to the total mass of the products.There
are many different types of chemical reactions, including combination reactions, decomposition reactions, single-replacement reactions, double-replacement reactions, and combustion reactions.
Classification of Matter: The Periodic Table: Classification Of Matter Pogil Answer Key Pdf
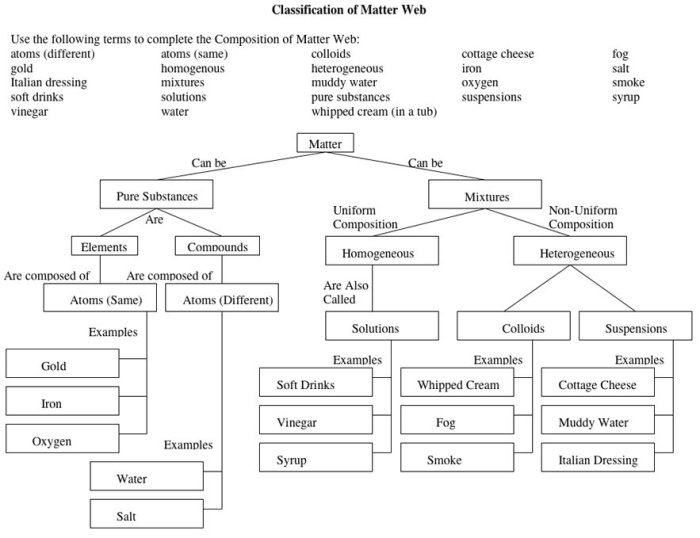
The periodic table is a tabular arrangement of the chemical elements. The elements are arranged in order of increasing atomic number, which is the number of protons in the nucleus of an atom.The periodic table is organized into 18 vertical columns, called groups, and 7 horizontal rows, called periods.
The groups are numbered 1-18 from left to right, and the periods are numbered 1-7 from top to bottom.The periodic table can be used to predict the properties of elements. For example, elements in the same group tend to have similar chemical properties.
Question Bank
What is the difference between a homogeneous and heterogeneous mixture?
A homogeneous mixture is one in which the components are evenly distributed throughout the mixture, while a heterogeneous mixture is one in which the components are not evenly distributed.
What are the three states of matter?
The three states of matter are solid, liquid, and gas.
What is a chemical reaction?
A chemical reaction is a process in which atoms or molecules are rearranged to form new substances.