Nomenclature worksheet 3 ionic compounds containing polyatomic ions – Embark on a journey of chemical nomenclature with Nomenclature Worksheet 3: Ionic Compounds Containing Polyatomic Ions. This comprehensive guide unveils the intricacies of naming these compounds, empowering you to decipher their intricate chemical structures.
Delve into the formation, examples, and naming conventions of ionic compounds containing polyatomic ions. Master the art of recognizing and interpreting these complex chemical entities, unlocking a deeper understanding of chemical interactions.
Ionic Compounds Containing Polyatomic Ions
Ionic compounds containing polyatomic ions are formed when a metal cation combines with a polyatomic anion. Polyatomic ions are ions that contain more than one atom. The charges of polyatomic ions are typically determined by the number of atoms in the ion and the charge of each atom.
For example, the sulfate ion (SO 42-) is a polyatomic ion that contains one sulfur atom and four oxygen atoms. The sulfur atom has a charge of +6, and each oxygen atom has a charge of -2. The overall charge of the sulfate ion is -2.
When a metal cation combines with a polyatomic anion, the charges of the ions must balance out. For example, the sodium cation (Na +) has a charge of +1, and the sulfate ion (SO 42-) has a charge of -2. To balance out the charges, two sodium cations must combine with one sulfate ion to form the ionic compound sodium sulfate (Na 2SO 4).
Naming Conventions for Ionic Compounds Containing Polyatomic Ions, Nomenclature worksheet 3 ionic compounds containing polyatomic ions
The naming conventions for ionic compounds containing polyatomic ions are similar to the naming conventions for ionic compounds containing simple ions. The name of the cation is written first, followed by the name of the anion. The name of the anion is changed to end in -ate or -ite, depending on the charge of the ion.
For example, the ionic compound Na 2SO 4is named sodium sulfate. The cation is sodium (Na +), and the anion is sulfate (SO 42-). The name of the anion is changed from sulfate to sulfate because the ion has a charge of -2.
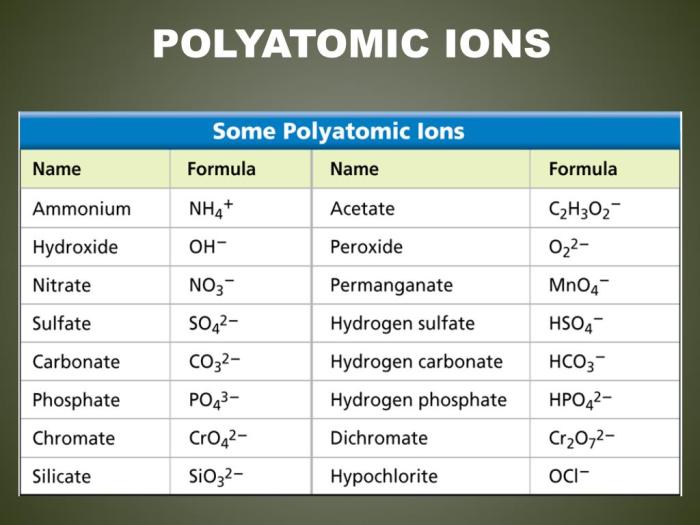
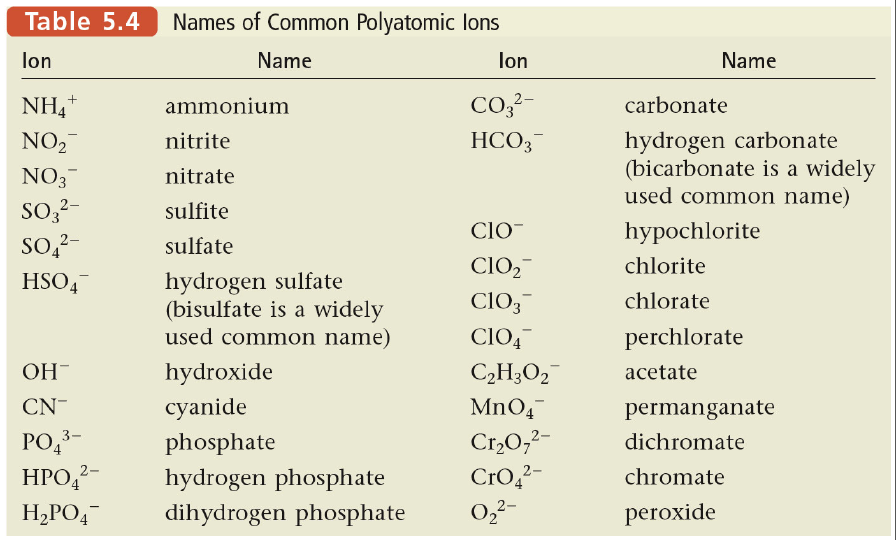
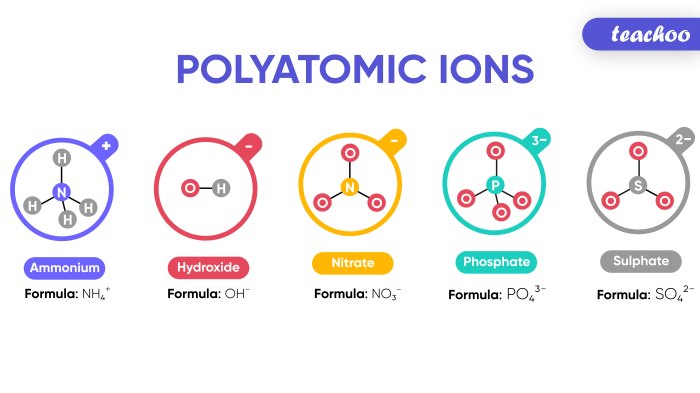
Polyatomic Ions
Polyatomic ions are ions that contain more than one atom. Polyatomic ions can be either positive or negative. The most common polyatomic ions are anions, which are negatively charged ions.
Some common polyatomic ions and their charges are listed below:
- Sulfate (SO 42-)
- Nitrate (NO 3–)
- Carbonate (CO 32-)
- Hydroxide (OH –)
- Ammonium (NH 4+)
Polyatomic ions are often used in naming ionic compounds. When naming an ionic compound containing a polyatomic ion, the name of the polyatomic ion is changed to end in -ate or -ite, depending on the charge of the ion.
Naming Ionic Compounds
The rules for naming ionic compounds are as follows:
- The name of the cation is written first, followed by the name of the anion.
- The name of the anion is changed to end in
- ate or
- ite, depending on the charge of the ion.
- If the metal cation can form more than one type of ion, the charge of the ion is indicated using Roman numerals in parentheses.
For example, the ionic compound Fe 2O 3is named iron(III) oxide. The cation is iron (Fe), and the anion is oxide (O 2-). The name of the cation is changed to iron(III) because the iron cation has a charge of +3.
The table below compares the naming of ionic compounds with and without polyatomic ions:
| Ionic Compound | Name |
|---|---|
| NaCl | Sodium chloride |
| Na2SO4 | Sodium sulfate |
| Fe2O3 | Iron(III) oxide |
| NH4Cl | Ammonium chloride |
FAQ Guide: Nomenclature Worksheet 3 Ionic Compounds Containing Polyatomic Ions
What are polyatomic ions?
Polyatomic ions are groups of atoms that carry a net electrical charge and behave as a single unit within ionic compounds.
How do I name ionic compounds containing polyatomic ions?
Follow these steps: 1) Identify the metal and polyatomic ion; 2) Write the metal’s name first, followed by the polyatomic ion’s name; 3) Use parentheses to enclose the polyatomic ion if it contains multiple atoms; 4) Balance the charges of the ions using subscripts.